Glycolysis: Thermodynamics and Predictions of Metabolic Pathways
Aim of this work is to thermodynamically describe single reaction steps of the glycolysis metabolic pathway for which current works fail to explain its occurrence.
Description
Thermodynamics has successfully been applied to many areas of biological systems. However, application of thermodynamics to a detailed examination of entire metabolic networks remains a challenging task. This is due to a scarcity of both, reliable thermodynamic data on metabolic reactions and rigorous models describing the physico-chemical properties of metabolites in mixtures. This project is a collaboration with the Department of Physical Chemistry from University of Rostock and the Department of Environmental Microbiology from Helmholtz-Centre for Environmental Research GmbH in Leipzig. The project aims at applying new accurate thermodynamic methods in order to improve metabolic pathway predictions. The procedure will be applied to the enzymatic reaction cascade of the glycolysis metabolism for which thermodynamics fails to explain its occurrence.
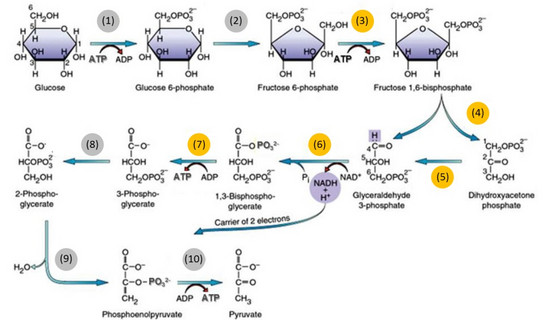
Main goal at the Laboratory of Thermodynamics is to understand the thermodynamics of reaction equilibria of the single reaction steps taking part in glycolysis. Therefore, the reaction equilibria will be measured in-vitro and influences of additives, such as macromolecular crowders and inorganic salts (both of which are present in-vivo), on reaction equilibria will be determined. Further, activity coefficients of the metabolites will be measured, in order to access deviations of the metabolites from their standard state, which are caused by interactions between all present compounds in the reaction mixture and thus have strong impact on reaction equilibrium position [1]. Additionally, the activity coefficients will be modelled with the electrolyte Perturbed-Chain Statistical Associating Fluid Theory (ePC-SAFT) proposed by Held et al. [2] using pure-metabolite and binary parameters. These predictions will be used for the determination of the standard Gibbs energy of reaction ΔRg0 for the single reactions marked in yellow in Figure 1.

References
| [1] | P. Hoffmann, C. Held, T. Maskow, and G. Sadowski: "A thermodynamic investigation of the glucose-6-phosphate isomerization" Biophys Chem, vol. 195, pp. 22-31, 2014. |
| [2] | C. Held, T. Reschke, S. Mohammad, A. Luza, and G. Sadowski: "ePC-SAFT revised" Chemical Engineering Research and Design, vol. 92, pp. 2884-2897, 2014. |