M. Sc. Marcel Klinksiek
Microfluidics for Structure-Reactivity Relationships Aided by Thermodynamics & Kinetics
The relationships between reactant structure, co-solvents, catalyst, and their respective reactivity in esterification processes is a research area that crosslinks thermodynamics, kinetics and chemical engineering. It is the goal of this project to predict the influence of solvent, concentration, and catalyst on the kinetics and equilibria of esterification reactions by thermodynamic modeling.
Description
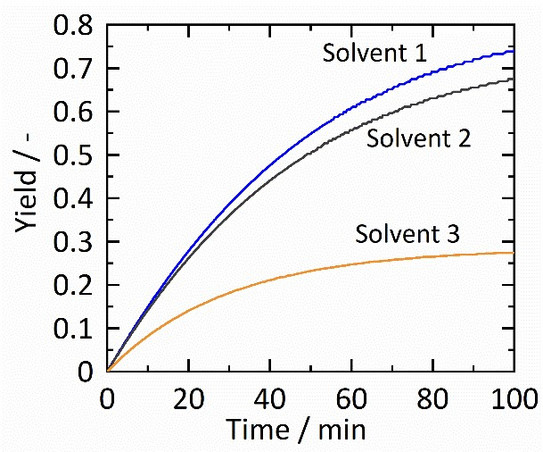
Levulinic acid (LA) attracts attention as LA its related products (esters) have a formidable potential to be used as a renewable feedstock material for chemical syntheses [1]. Thermodynamics allows predicting the effects of chemical solvents on the reaction in order to link the structure of the reaction partners with the efficiency of the reaction in terms of kinetics and maximum product yield. The aim of this work is to develop methods to predict the efficiency of the chemical reactions with varying chemical solvents for LA and LA-based compounds and their derived products. The influence of different solvents on the reaction equilibrium and kinetic is exemplarily shown in Figure 1.
The reaction equilibria and kinetics of the esterification reactions are measured under influence of co-solvents to provide a database for the model development. Activities of the reactants and products will be predicted based on the experimental kinetic profiles by means of the equation of state ePC-SAFT. ePC-SAFT is used for this step since it allows accurately characterizing interactions in complex media involving charge effects [2].
References
| [1] | J. Horvat, B. Klaić , B. Metelko, & V. Šunjić. "Mechanism of levulinic acid formation" Tetrahedron letters. 1985, 26(17), 2111-2114. |
| [2] | M. Bülow, M. Ascani, & C Held. "ePC-SAFT advanced-Part I: Physical meaning of including a concentration-dependent dielectric constant in the born term and in the Debye-Hückel theory" Fluid Phase Equilibria. 2021, 535, 112967. |