M. Sc. Espen Fritschka
Phase Behavior of Drug Delivery Systems as Function of pH and Salt Concentration
The bioavailability of an active pharmaceutical ingredient (API) is often limited by its poor water solubility, which is strongly influenced by pH and the presence of salts. This project develops an adequate model to predict the phase behavior of APIs in water and in (lipid-based) drug delivery systems.
Description
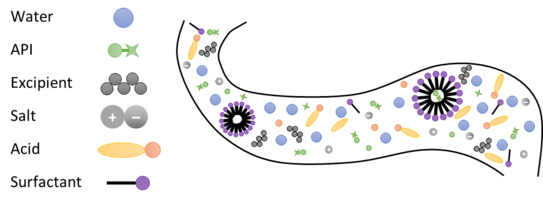
The gastrointestinal fluids contain, besides water and lipids, other components like ions, salts and acids, which contribute to the observable phase behavior tremendously [1]. pH and salt concentrations in the human body strongly depend on the location (gastric or intestinal system) and the time of intake (saturated or fasted state). The complex interplay between all components present determines the APIs bioavailability.The gastrointestinal fluids contain, besides water and lipids, other components like ions, salts and acids, which contribute to the observable phase behavior tremendously [1]. pH and salt concentrations in the human body strongly depend on the location (gastric or intestinal system) and the time of intake (saturated or fasted state). The complex interplay between all components present determines the APIs bioavailability.
This project aims to consider these effects with a more suitable theoretical model to predict the phase behavior more precisely. Especially the influence of the corresponding pH and different salts is decisive for the bioavailability, whereas the difference between fasted- and fed-state, respectively, must be taken into account.
References
| [1] | L. Klumpp, J. Dressmann: “Physiologically based pharmacokinetic model outputs depend on dissolution data and their input: Case examples glibenclamide and dipyridamole” Eur. J. Pharm. Sci., Vol. 151, p. 105380, 2020 |