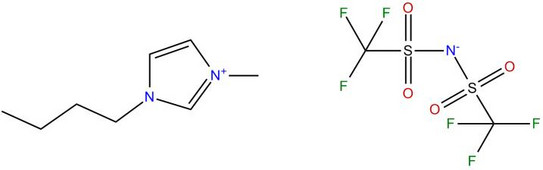
Thermodynamics of Non-Aqueous and Pure-Ionic Electrolyte Solutions
The aim of this work is the further development of ePC-SAFT towards quantitative model predictions for mixtures containing ionic liquids.
Description
Applications
ILs are considered suitable for a variety of applications in the (bio)-chemical industry. One major focus is their use as extraction agents in the downstream process for mixtures with temperature-sensitive chemicals. Therefore, the knowledge of liquid-liquid equilibria (LLE) with classical solvents is of great importance; hence, such phase behavior will be investigated in this study. A second possible area of application is the use of ILs in ionothermal synthesis. In the isothermal synthesis, ILs are applied as a reaction media for the production of e.g. metal nanoparticles. This technology requires knowledge on solubility of precursors in ILs as well as the influence of ILs on reaction equilibria and reaction kinetics. Thus, this project is focused on solubility in ILs and use of ILs as solvent in (bio)-catalyzed reactions. The influence of the IL on the equilibrium and thus on the conversion will be predicted with ePC-SAFT, which will finally allow a solvent screening prior to experimental validation.
ePC-SAFT modelling
To model the ILs by an ion-specific approach, the equation of state ePC-SAFT (electrolyte Perturbed-Chain Statistical Associating Fluid Theory) will be further developed and applied to model phase equilibria and reaction equilibria in mixtures with ILs. It is the ultimate goal to replace as much of time-consuming experimental work by quantitative correct ePC-SAFT predictions in order to have the ability of a model-based IL selection for a specific task for separations and chemical syntheses.