M. Sc. Alexander Strangmann
Influence of coating materials on ASD stability
Many active pharmaceutical ingredients (APIs) suffer from low aqueous solubility, limiting their bioavailability. Bioavailability can be improved by dissolving the API in a polymer, yielding so-called amorphous solid dispersions (ASDs). This project aims at investigating the influence of coating materials, temperature and relative humidity (RH) on the stability of coated ASDs during storage.
Description
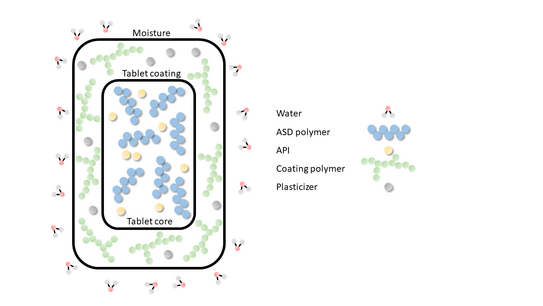
ASDs are often thermodynamically unstable, resulting in the eventual formation of poorly water-soluble API crystals. API crystallization defeats the bioavailability improvement of an ASD and is therefore highly undesirable. However, at temperatures below the glass transition temperature of the ASD, crystallization is kinetically prevented by low molecular mobility.
Higher storage temperatures and the absorption of lower molecular substances like water from air increase molecular mobility within the ASD [2]. Furthermore, ASD tablets are often coated with an additional polymer to improve their appearance and swallowability. Plasticizers are necessary ingredients to create such coatings. These plasticizers may also increase molecular mobility and thus induce API crystallization.
This project investigates the interplay of effects resulting from storage temperature, RH and coating materials on ASD stability.
References
| [1] | T. Vasconcelos, B. Sarmento, P. Costa: "Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs" Drug discovery today 12 (23-24), S. 1068–1075, 2007. |
| [2] | A. Newman, G. Zografi: "What are the important factors that influence API crystallization in miscible amorphous API–excipient mixtures during long-term storage in the glassy state?" Molecular Pharmaceutics 19 (2), S. 378-391, 2021. |